Jeffrey N. Johnston
Stevenson Professor of Chemistry
Dr. Johnston will be accepting graduate students for Fall 2026.
|
Since 1999, we have been uncovering new modes of reactivity and applying these discoveries in chemistry to the development of fundamentally new concepts in enantioselective catalysis and basic reaction development. Complex natural product total synthesis is the beginning and end of the project development cycle, and we have more recently succeeded in applying our discoveries to the de novo synthesis of therapeutics. Our accomplishments span across the areas of target synthesis, therapeutic development, and enantioselective catalysis. With several colleagues in biochemistry, cancer biology, and drug development, we collaboratively advance the discipline of chemical biology to leverage the power of chemical synthesis, thereby clarifying our basic understanding of the pathology and treatment of disease. |
Specializations
|
VICB |
Expertise | chemical synthesis with an emphasis on chiral amines and heterocycles; catalysis with and emphasis on enantioselective bifunctional catalysis using hydrogen bonding; total chemical synthesis with an emphasis on alkaloid and unusual peptide natural products; therapeutic development with an emphasis on heterocyclic small molecule-enabling studies of disease; training with an emphasis on experimental synthetic organic chemistry knowledge and research in preparation for professional careers in the pharmaceutical and biotechnology industries.
Overview | Advances in chemical synthesis are enabled by the discovery of new activation and reactivity paradigms that add versatility to existing chemical feedstock. Complexity-building reactions convert inexpensive raw materials into structurally and functionally complex small molecules capable of selective interactions with biological molecules. These small molecules are prized for their potential as therapeutics to cure disease and improve quality of life. Our expertise in complex small molecule synthesis spans existing chemotypes, particularly alkaloids and peptides, but we forage in areas of chemical space not yet explored. Our devotion to efficiency and complexity in chemical synthesis provides training for future medicinal and process chemists, and their allies in the fight against disease. Our creative strategies and innovative methods advance the field of synthesis and catalysis, often through discoveries that change the way we think about chemistry.
Complex natural (and unnatural) product synthesis | Small molecule complexity is, and always will be, the foundation of therapeutic development, but synthesis uses this platform as a springboard to more potent and selective tools. In our portfolio of total synthesis, we have reported concise preparations of complex heterocyclic alkaloids serratezomine A and hapalindoles K, A, and G. Our recent work has moved beyond the practice of simply targeting the natural material to include molecularly edited versions (e.g. fluorinated derivatives). These strategic decisions and commitments to provide unique materials create unusual opportunities for collaboration.
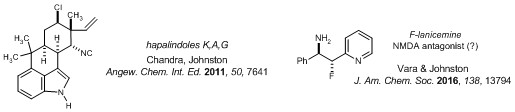
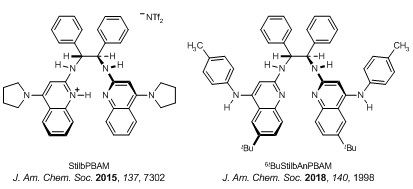
Enantioselective catalysis | Hydrogen bonds are pervasive determinants of order and function in biological settings, but have been used in enantioselective synthesis in vitro for little over a decade. Heterocyclic, Bis(AMidine)-based (BAM) catalysis with strong acid co-additives provide unique polar ionic hydrogen bond functionality, complementing the more pervasive polar covalent hydrogen bond catalysts (e.g. diol, urea, phosphoric acid). BAM catalysis has considerably deepened the utility of the aza-Henry reaction while establishing it as a gateway to single enantiomer amines and diamines. Successful translation of BAM catalysis from azomethine (C=N) to alkene (C=C) activation was achieved in the context of alkene halofunctionalization. A highlight of this transition is the enantioselective carbon dioxide-fixation reaction of homoallylic alcohols, providing the first metal-free variant of its type, and establishing the feasibility of CO2-activation using weak hydrogen bond donor-acceptor catalysis.
Therapeutic development | New reactions and reagents can render otherwise inaccessible complex small molecules with therapeutic potential available in quantities needed for development in animal models of disease. This cycle of discovery and application is a hallmark of our Vanderbilt years, often involving heterocyclic targets. Our collaborations are driven equally by advances in chemistry as they are by the study of a disease. As a result, our track record in collaboration spans oncology, infectious disease, neuroscience, and most recently, cardiovascular pharmacology.

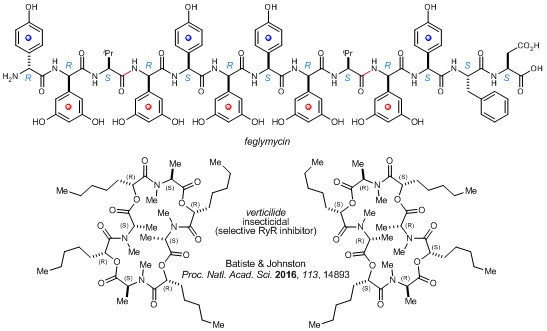
Peptide synthesis | Peptide, peptidic, and peptidomimetic therapies have burgeoned in the past decade, stimulated by advances in drug delivery and challenging, but increasingly tractable, targeted ligand-receptor interactions and disease pathways. Enabled by a discovery in basic amide bond synthesis (Umpolung Amide Synthesis: UmAS), we have streamlined peptide synthesis involving unnatural amino amides by circumventing the active ester intermediate common to all known amide synthesis reactions. This approach provided a new field of opportunity for enantioselective synthesis of nitroalkane derivatives, and it also laid a path to the largest, most complex peptides we have prepared (>2000 MW). Currently, we are advancing on an exciting new hypothesis that cyclic oligomeric depsipeptides might be unique tools for interrogating molecular interactions.
Training | Through these studies, the student is trained how to think about problems in organic chemistry from an approach that involves extensive laboratory experimentation. Germane to this training is the routine use of spectroscopic (NMR, IR, mass spectroscopy, X-ray diffraction) and analytical (chiral stationary phase HPLC) techniques. Many of these projects require the student to draw knowledge from an extensive body of literature in organic chemistry while developing truly novel reactions and the reagents (catalysts) that promote them with high levels of stereocontrol. Furthermore, our approach to the development of new chemistry follows a highly synergistic combination of informed design, mechanistic organic chemistry, empiricism, and at times, a touch of luck.
Representative Publications
|
orcid.org/0000-0002-0885-636X |
|
“The Backbone Constitution Drives Passive Permeability Independent of Side Chains in Depsipeptide and Peptide Macrocycles” Madelaine P. Thorpe, Abigail N. Smith, Daniel J. Blackwell, Corey R. Hopkins, Bjorn C. Knollmann, Wendell S. Akers, and Jeffrey N. Johnston* Chem. Sci. 2024 “Backbone-Determined Antiarrhythmic Structure-Activity Relationships for a Mirror-Image, Oligomeric Depsipeptide Natural Product” Madelaine P. Thorpe, Daniel J. Blackwell, Bjorn C. Knollmann, and Jeffrey N. Johnston* J. Med. Chem. 2024, 67, 12205–12220. "Generality-Driven Catalyst Development: A Universal Catalyst for Enantioselective Nitroalkene Reduction” Zihang Deng, Melanie A. Padalino, Julius E. L. Jan, Sangjun Park, Michael W. Danneman, and Jeffrey N. Johnston* J. Am. Chem. Soc. 2024, 146, 1269-1275. “ent-Verticilide B1 inhibits type 2 ryanodine receptor channels and is antiarrhythmic in Casq2-/- mice” Aaron Gochman, Tri Q. Do, Kyungsoo Kim, Jacob A. Schwarz, Madelaine P. Thorpe, Daniel J. Blackwell, Abigail N. Smith, Wendell S. Akers, Razvan L. Cornea, Derek R. Laver, Jeffrey N. Johnston, Bjorn C. Knollmann, submitted 7/3/23 bioRxiv 2023, 2023.07.03.547578 Mol. Pharm. 2024, 105, in press. "RyR2 Binding of an Antiarrhythmic Cyclic Depsipeptide Mapped Using Confocal Fluorescence Lifetime Detection of FRET.” Jaroslava Seflova#, Jacob A. Schwarz#, Abigail N. Smith#, Bengt Svensson, Daniel J. Blackwell, Taylor A. Phillips, Roman Nikolaienko, Elisa Bovo, Robyn T. Rebbeck, Aleksey V. Zima, David D. Thomas, Filip Van Petegem, Björn C. Knollmann, Jeffrey N. Johnston*, Seth L. Robia, and Răzvan L. Cornea* ACS Chem. Biol. 2023, 18, 2290–2299. “The selective RyR2 inhibitor ent-verticilide suppresses atrial fibrillation susceptibility caused by Pitx2 deficiency” Kyungsoo Kim, Daniel J. Blackwell, Madelaine P. Thorpe, Jeffrey N. Johnston, Bjorn C. Knollmann* J. Mol. Cell. Cardiol. 2023, 180, 1-9. “In vivo pharmacokinetic and pharmacodynamic properties of the antiarrhythmic molecule ent-verticilide” Daniel J. Blackwell, Abigail N. Smith, Tri Do, Aaron Gochman, Jeffrey Schmeckpeper, Corey R. Hopkins, Wendell S. Akers,* Jeffrey N. Johnston,* Bjorn C. Knollmann* J. Pharm. Exptl. Ther. 2023, 385, 205-213. “Enantioselective Synthesis of cis- and trans-Cycloheptyl β-Fluoro Amines by Sequential aza-Henry Addition/Ring-Closing Metathesis” Bing, J. A.; Johnston, J. N.* Org. Lett. 2023, 25, 950. PMC10240541 “Secondary Orbital Effect Involving Fluorine is Responsible for Substrate-Controlled Diastereodivergence in the syn-aza-Henry Reaction of α-Fluoronitroalkanes” Smajlagic, I.; Johnston, J. N.,* Dudding, T.* Chem. Eur. J. 2023, 29, e202204066. NIHMSID 1904292 “Structure-Activity Relationships for the N-Me- versus N-H-Amide Modification to Macrocyclic ent-Verticilide Antiarrhythmics” Smith, Abigail; Thorpe, Madelaine; Blackwell, Daniel; Batiste, Suzanne; Hopkins, Corey; Schley, Nathan; Knollmann, Bjorn; Johnston, Jeffrey ACS Med. Chem. Lett. 2022, 13, 1755-1762. PMC9661706 “Preparation of N-Aryl Amides by Epimerization-Free Umpolung Amide Synthesis” Crocker, M. S.; Deng, Z. Johnston, J. N. J. Am. Chem. Soc. 2022, 144, 16708-16714. PMC9634722 “Enantioselective Iodolactonization to Prepare ε-Lactone Rings Using Hypervalent Iodine” Payne, J. L.;† Deng, Z.;† Flach, A. L.;† Johnston, J. N.* [†equal contribution] Chem. Sci. 2022, 13, 7318-7324. PMC9214890 “Resolving Bromonitromethane Sourcing by Synthesis: Preparation at the Decagram Scale” Thorpe, M. P.; Smith, A. N.; Crocker, M. S.; Johnston, J. N.* J. Org. Chem. 2022, 87, 5451. PMC9109156 “Exercise Causes Arrhythmogenic Remodeling of Intracellular Calcium Dynamics in Plakophilin-2 Deficient Hearts” van Opbergen, C.; Bagwan, N.; Maurya, S; Kim, J. C.; Smith, A. N.; Blackwell, D. J.; Johnston, J. N.; Knollmann, B.; Cerrone, M.; Lundby, A.; Delmar, M. Circulation 2022, 145, 1480-1496. PMC9086182 “Fluorine-Induced Diastereodivergence Discovered in an Equally Rare Enantioselective syn-aza-Henry Reaction” Bing, J. A.; Schley, N. D.; Johnston, J. N.* Chem. Sci. 2022, 13, 2614-2623. PMC8890141 “Ring-Size as an Independent Variable in Cyclooligomeric Depsipeptide Antiarrhythmic Activity” Smith, A. N.; Blackwell, D. J.; Knollmann, B. C.; Johnston, J. N. ACS Med. Chem. Lett. 2021, 12, 1942-1947. PMC8667307 |




