Vanderbilt biologists discover genetic pathways linking the immune and circulatory systems of mosquitoes during infection
By Andy Flick, Evolutionary Studies scientific coordinator
Vanderbilt biologists have discovered the genetic pathways that link the immune and circulatory systems of mosquitoes during the fight against infection. A mosquito fighting infection of malaria or bacteria attracts immune cells to its heart that filter microbes that are flowing in its blood, called hemolymph. The discovery of two pathways that link immunity and hemolymph circulation is a major contribution to the understanding of how mosquitoes, which are themselves disease vectors, respond to infection.
Julián F. Hillyer, professor of biological sciences, and his research team investigate the physiology of mosquitoes and, specifically, how the mosquito heart pumps hemolymph, how its immune cells fight infection and how these processes interact.
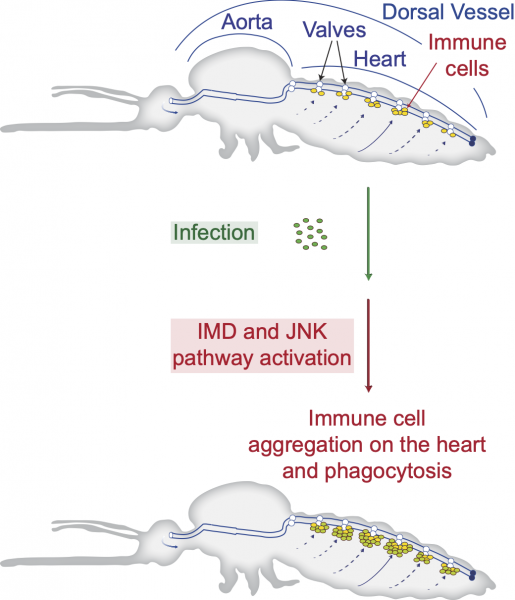

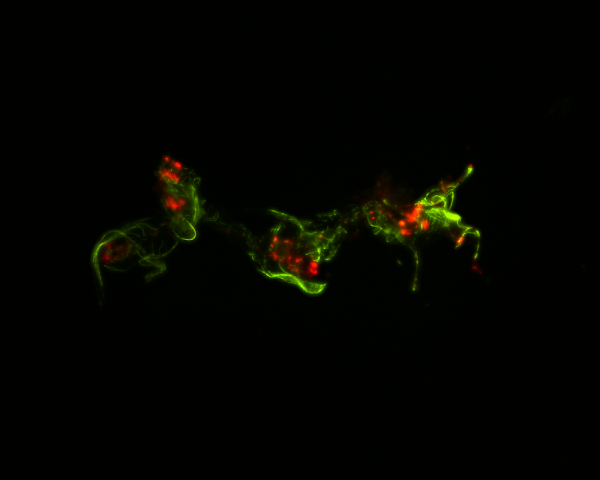
In some cases, mosquitoes beat infection through immunologically active cells in the heart. Because the circulatory system of mosquitoes contains only one contracting vessel and no arteries or veins, their hemolymph flows freely throughout the body. “Insects deploy a powerful immune response against infection, with immune cells aggregating on the heart to destroy passing microbes. Yet, the genetic mechanisms that guide these immune cells to the heart remained unknown,” said Yan Yan, PhD’21, a lead researcher on the project.

Hillyer’s team studied the expression of every gene in the mosquito heart and identified the genetic pathways that become significantly activated on the heart when an infection occurs. By disrupting these pathways using a technique called RNA interference, the researchers discovered how the genetic pathways are involved.
The immune deficiency pathway, which produces and secretes immune proteins, also recruits free-floating immune cells to settle on the heart. The team also found that a pathway that plays a role in longevity, reproduction and defense brings immune cells to the heart. Immune cells that arrive on the heart eat and digest microbes, increasing a mosquito’s likelihood to overcome infection.
“This research could lay the foundation for novel strategies that protect beneficial insects or harm detrimental ones.” – Julián F. Hillyer
Hillyer, Yan and their team hypothesize that the genetic pathways identified in this study drive immune responses on the hearts of other insects—including disease vectors, agricultural pests and crop pollinators—because the immune and circulatory systems are functionally integrated throughout the insect tree of life and are evolutionarily conserved in the insect lineage.
“This research could lay the foundation for novel strategies that protect beneficial insects or harm detrimental ones,” Hillyer added.
To continue this advance in knowledge, Hillyer’s team is investigating how pathway disruption affects insect heart contractions and is looking for heart-specific factors that act as “homing signals” for the arrival of immune cells on the heart.
The article, “The immune deficiency and c-Jun N-terminal kinase pathways drive the functional integration of the immune and circulatory systems of mosquitoes” was published in the journal Open Biology on Sept. 7.
The work was conducted in the department of biological sciences and the Evolutionary Studies Initiative at Vanderbilt University. The research was funded by National Science Foundation grants IOS-1456844 and IOS-1949145. Members of the research team include first author Leah Sigle, PhD’18, postdoctoral scholar David Rinker and research assistant Tania Estévez-Lao. Former Vanderbilt faculty member and current associate professor of epidemiology and biostatistics at University of California at San Francisco John A. Capra also participated in the research.